HyTriage (汇创宜), a healthcare service provider that offers chronic disease detection and management platform, has announced lately that it has scooped up ¥25 million (approximate US$4 million) in a new funding round from Yahui Medical Fund. The capital will be mainly used for market expansion and product refinement. The company is said to have raised ¥10 million (approximately US$1.5 million) from a Series A round in 2015 from CSC Group, whose shares are traded on “The New Third Board” (National Equities Exchange And Quotations).
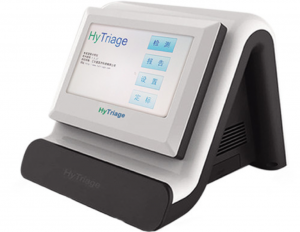
Founded in 2013, HyTriage focuses specifically on developing and supplying POCT (point-of-care testing: diagnostic testing conducted at the time and place of patient care, instead of specialized medical laboratories) devices. Its flagship product is Colloidal Gold-Labeled Quantitative Immunoassay Analyzer, which is intended primarily for grassroots medical institutions, including community health centers, county-level hospitals and community hospitals.
In China, medical institutions at different tiers, which are subject to the “multi-tiered medical care system” introduced by the government in light of the seriousness and urgency, as well as the difficulty, to treat certain diseases, are tasked with the responsibilities of treating different diseases. And this has in part led to the swelling of grassroots medical institutions. By the end of June 2016, medical institutions across China had climbed to 989,000, among which 93.7% are grassroots medical institutions, according to statistics published by the National Health and Family Planning Commission of the People’s Republic of China. And one thing that these grassroots medical institutions are in urgent need of is diagnostic products for common diseases.
This is basically why the company made its foray into this business. Unlike traditional colloidal gold reagents manufacturers, which produce both analyzers and test cards only to find that their solutions are only applicable for a limited few diseases, HyTriage has opted to produce only analyzers, with the test cards being provided by its selected suppliers.
In this way, the company can, firstly, offer solutions applicable for a wide array of diseases by integrating production resources and, secondly, deliver solutions with high quality and competitive price to better meet the specific and diversified diagnostic requirements of grassroots medical institutions.
The company has so far inked partnerships with over 10 reagents manufacturers and extended the list of diseases that can be tested with its analyzer to more than 30, among which include the common diseases and some chronic diseases oftentimes identified in children and the elderly. More importantly, the price for each diagnostic test has been brought down to less than ¥100 (approximately US$15) with the company’s analyzer.
According to the team, the company has, up till now, deployed in total 7,500 analyzers in 15 provinces. And its analyzers are mainly installed in grassroots medical institutions in third and fourth-tier cities. The company has also ushered in some Grade A hospitals, which need its analyzer for quick diagnostic tests in their clinical departments (for example, emergency department).
The company has now largely broken even. In 2018, the company looks to further expand its market share, upgrade its product with information technologies and enhance user experience.
