This story originally appeared in Open Source, our weekly newsletter on emerging technology. To get stories like this in your inbox first, subscribe here.
Anything Elon Musk gets involved with or discusses tends to court controversy, and Neuralink is no exception. The neurotechnology company recently received the green light from the US Food and Drug Administration (FDA) to embark on human clinical trials with its N1 implant, igniting a mix of anticipation and apprehension.
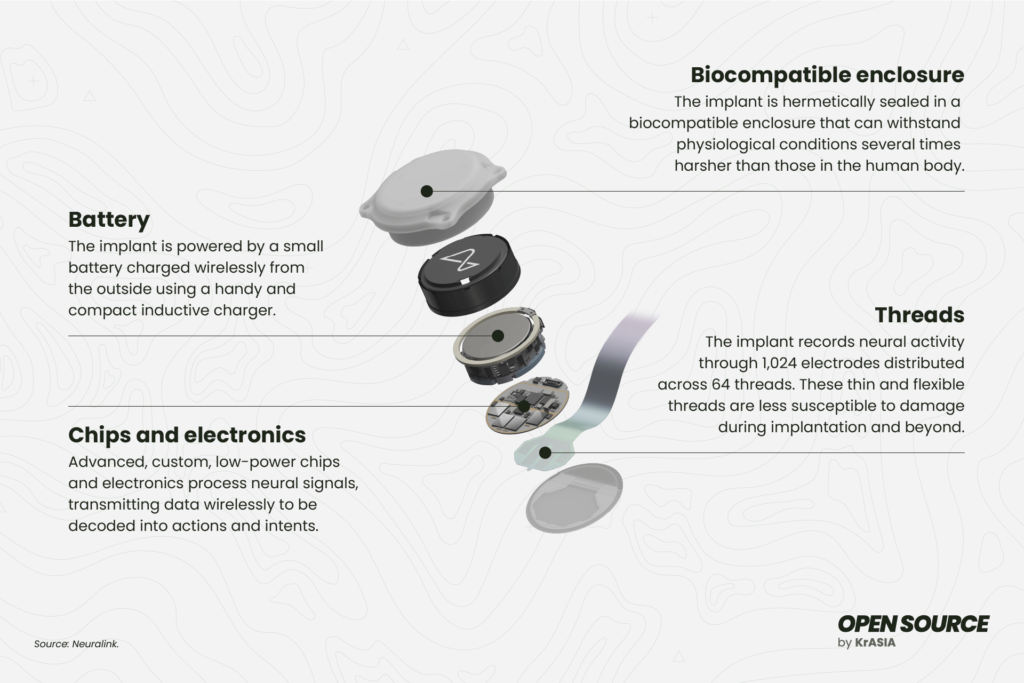
Neuralink’s N1 implant
The N1 implant falls into the category of brain-computer interfacing (BCI) devices. Sized at approximately 23 by 8 millimeters, this compact, coin-sized implant is designed to facilitate a brain-computer interface (BCI) when embedded within the human brain.
BCIs typically involve acquiring electrical signals from the brain, interpreting them, and potentially transforming them into actions. This technology can potentially be utilized in a wide range of applications, with the present focus being on health and medical uses. For example, BCIs could offer a pathway to restore physical mobility for individuals with disabilities, providing an alternative route for neurons to communicate with affected limbs or body parts. This use case is also widely considered the foundation of the industry, with various researchers making strides in this direction to varying degrees.
According to Neuralink’s descriptions, the N1 implant will offer an all-day battery life and it will be wireless rechargeable. Its design will also enable it to be flush with the skull, rendering it cosmetically invisible upon implantation.
While the prospect of human clinical trials involving BCI implants can be unsettling, it’s crucial to recognize the limitations set forth in the FDA’s approval for Neuralink. According to media reports, this phase of trials will be tightly regulated, with specific conditions that the neurotechnology company must adhere to. Notably, these trials will only be accessible to individuals afflicted by quadriplegia resulting from injury or amyotrophic lateral sclerosis (ALS), a condition marked by the degeneration of nerve cells in the brain and spinal cord.
Dubbed the PRIME study, which stands for “precise robotically implanted brain-computer interface,” the objectives of this trial go beyond assessing the functionality of the N1 implant. Neuralink also plans to concurrently evaluate the safety and efficacy of the R1 robot, the surgical device responsible for implantation, and the N1 user app, the software facilitating the connection to the implant and the translation of brain signals into computer commands.

Rife with controversy
While Musk’s involvement alone is sufficient to garner widespread attention (and criticism), Neuralink’s endeavors provoke controversy for reasons beyond the persona of Musk, with several key factors at play.
One of these factors has been Neuralink’s testing practices, which have been widely scrutinized amidst allegations of animal cruelty. According to Reuters, over 1,500 animals have died in Neuralink’s labs since the company began experimenting on them in 2018. While the death of animal test subjects is not uncommon, the mortality rate has ostensibly been higher than usual due to an arduous development schedule. Neuralink employees have alleged that this timeline has led to more errors. Notably, a trial involving a seven-year-old monkey left the animal with “severe neurological defects” after leaked adhesive from the implant inflamed parts of its brain.
Another factor to consider is concerns regarding the technology itself. It is worth noting that Neuralink’s efforts to secure FDA approval were far from straightforward, with Musk publicly claiming at least on four occasions since 2019 that the company was on the verge of a breakthrough, only to renege on his promises later.
The FDA’s hesitance to grant approval stems primarily from uncertainty about the long-term effects of brain implants like the N1. Specifically, there was and still is ambiguity regarding the risk of permanent brain damage resulting from prolonged exposure to the implant and its battery.
There were also concerns that the implant might be challenging to remove, that wires could shift to different areas of the brain, and that the chips could overheat. Li Xiaojian, a researcher at the Chinese Academy of Sciences (Shenzhen Institutes of Advanced Technology), told 36Kr that when an electrode malfunctions, removing it from brain tissue is akin to inserting a needle into tofu and trying to extract it. The N1 implant employs 1,024 electrodes. Should any of these electrodes malfunction, removal or replacement would constitute a maneuver with significant risk of damaging the brain’s cortex.
On a broader level, ethical considerations about BCIs also come into play. Approaching a reality where collecting and deciphering brain signals is feasible creates concern over potential intruders eavesdropping on the human mind, and the possible risk of algorithms exerting control over the body, particularly in light of recent advancements in artificial intelligence.
All in your head
Over the years, Musk has been outspoken about Neuralink’s vision. The company’s initial goal is to establish facilities for surgical insertions of in-brain devices like the N1 implant, facilitating potential cures for neurological conditions such as obesity, autism, depression, and schizophrenia.
This first step would set the stage for more ambitious objectives, such as using BCIs to browse the internet or establishing telepathic communication between individuals. Ultimately, Neuralink envisions turning humans into cyborgs capable of defending against AI-powered sentient machines, with the prospects of consciousness transfer and even digital immortality not entirely ruled out. Whether any of these will eventually come to pass, however, remains uncertain.
It’s easy to become enthralled by Neuralink’s grand vision amidst the charisma of Musk. Nevertheless, judging by the trajectory of the broader industry, a gaping distance still exists between that dystopian future which Musk envisions and our current reality.
While neuroprosthetics, such as cochlear implants, have achieved varying degrees of success in helping the deaf regain their sense of hearing, progress in other facets of the industry has been notably sluggish.
Aside from Neuralink, research teams worldwide have spent years exploring the use of implants and devices like the N1 to treat conditions such as paralysis and depression. The key players in this field primarily remain in the earlier stages of development, typically ranging from Series A to B, indicating that commercialization is still some distance away. For example, Blackrock Neurotech, an industry veteran from the US, never managed to secure regulatory approval to commercialize its Utah Array, which it invented as far back as 1989.
Slow progress, however, does not equate to no progress. While companies like Neuralink are laying the groundwork, entities from other countries are also strengthening their capabilities in this field, with China poised to play a more prominent role in the coming years. In July 2017, Beijing announced a state-backed plan to achieve a first-mover advantage in the development of AI by 2030. The country is aiming to accomplish this by advancing research in three steps across the domains of neuroscience and AI, emphasizing three core areas. BCI is one of them.
This plan has since catalyzed the growth of the domestic industry, giving rise to notable players such as NeuroXess and Stairmed, each developing technologies seemingly comparable to Neuralink’s products.
With these developments in mind, one thing seems certain: given the impetus created by Musk and the emergence of other participants in the US, China, and other regions, advanced neurotechnology, including BCI devices like the N1 implant, is likely to materialize in the near future. Stay woke.

